Validation of the laboratory-specific conversion factor for BCR::ABL1 p210
The standardization of BCR::ABL1 quantification according to the international scale (IS) is of great importance for managing chronic myeloid leukemia (CML). The laboratory-specific conversion factor required for this is redetermined annually during quality assurance rounds. Over the last five years, MLL has been a member of the EUTOS project, in which, among other things, a procedure was developed to allow each and every laboratory to determine its own conversion factor in the future. We have collected the most important results of the project for you plus its associated future innovations.
Monitoring molecular response using reliable BCR::ABL1 quantification is essential for patients with chronic myeloid leukemia (CML) who are undergoing tyrosine kinase inhibitor therapy. The standardization of BCR::ABL1 quantification according to the international scale (IS) is of particular importance for managing the disease in each patient. It is used to compare the analytical results with the guidelines and recommendations of the European Leukemia Network (ELN) while also enabling the results to be compared between different laboratories.
MLL has been setting the standardized value BCR::ABL1IS since 2011, which is calculated as follows:
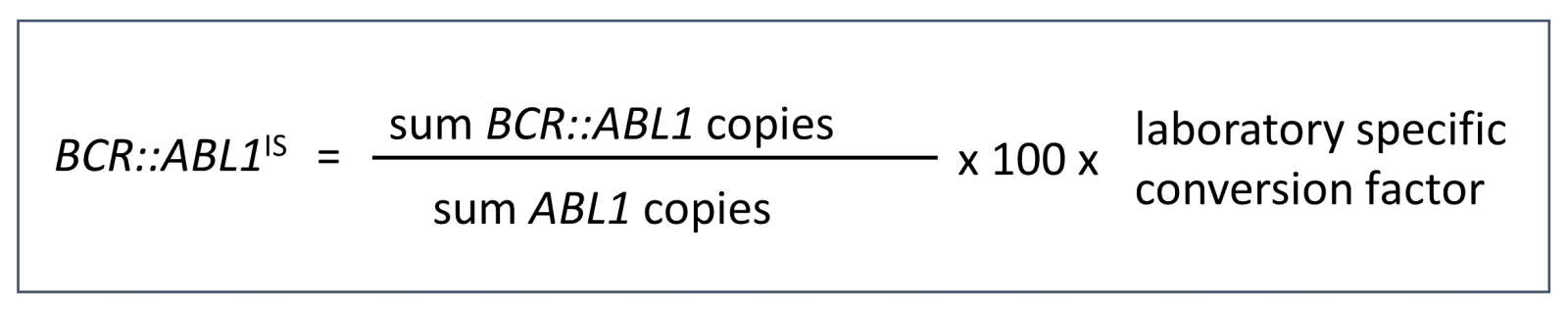
The laboratory-specific conversion factor is determined annually during a quality assurance round. Until now, this has involved the time-consuming exchange of samples with other reference laboratories. Between 2016 and 2021, MLL was a member of the EUTOS (EUropean Treatment and Outcome Study) project, an initiative of ELN and Novartis Oncology.
The main objectives of the study were:
- Optimization of current examination methods in CML
- Improvement of therapeutic outcomes
- Standardization of molecular follow-up in CML therapy
- Attainment of universal comparability of findings between different laboratories
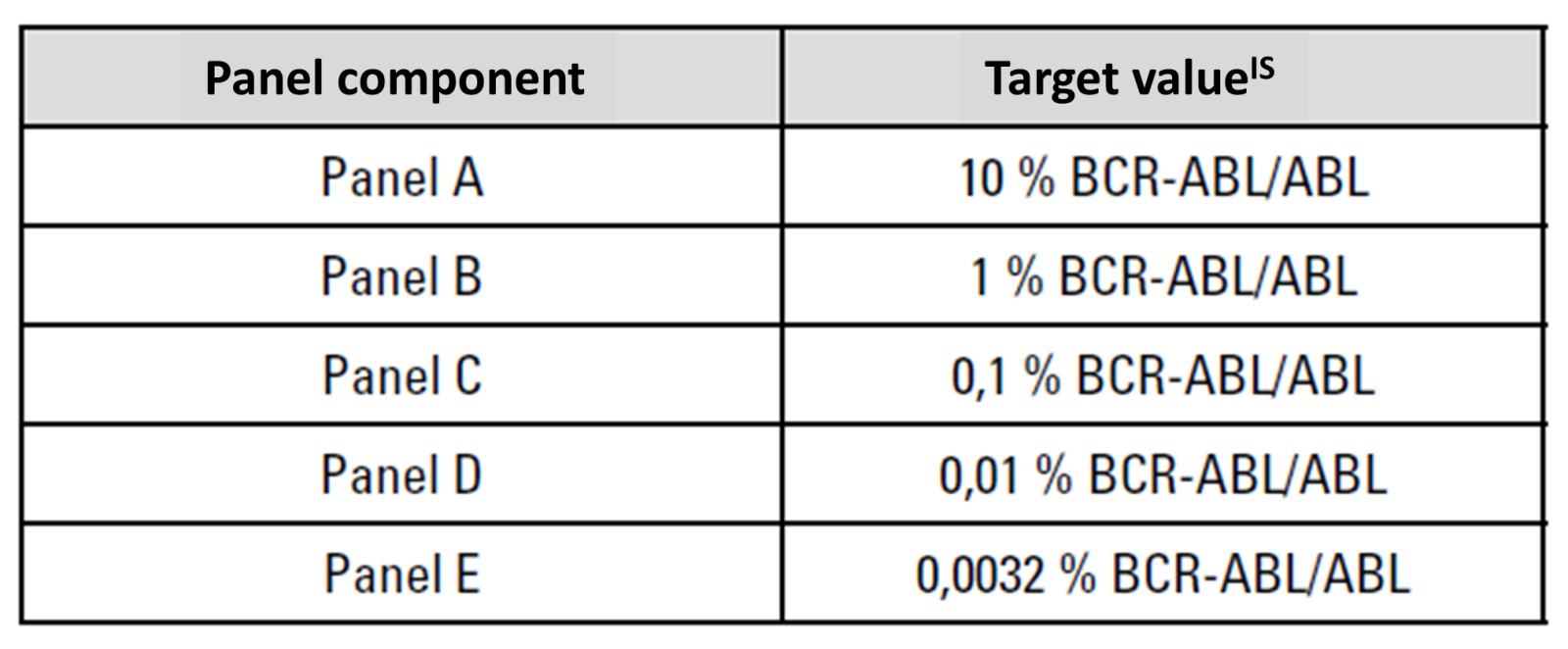
Among other things, the EUTOS project investigated the use of a secondary BCR::ABL1 reference panel based on lyophilized cells for determining and validating IS conversion factors. This reference material is traceable to the primary WHO international genetic reference panel for quantifying BCR::ABL1 translocation (Cross et al., Leukemia 2016). It consists of five components, each containing lyophilized cells that correspond to a specific BCR::ABL1 expression level (Figure 1).
Results of the EUTOS project (excerpt):
- Secondary reference panels can be used successfully for identifying and validating conversion factors.
- The use of secondary reference panels is equivalent to exchanging samples and is significantly less complex.
- Using secondary reference panels is recommended for independent validation of the laboratory-specific conversion factor.
The examination panel used in the study is now commercially available
and can be obtained by laboratories for self-validation of their conversion
factor (currently, the only supplier is AcroMetrix™ BCR::ABL1
Panel, Thermo Fisher Scientific, Figure 1).
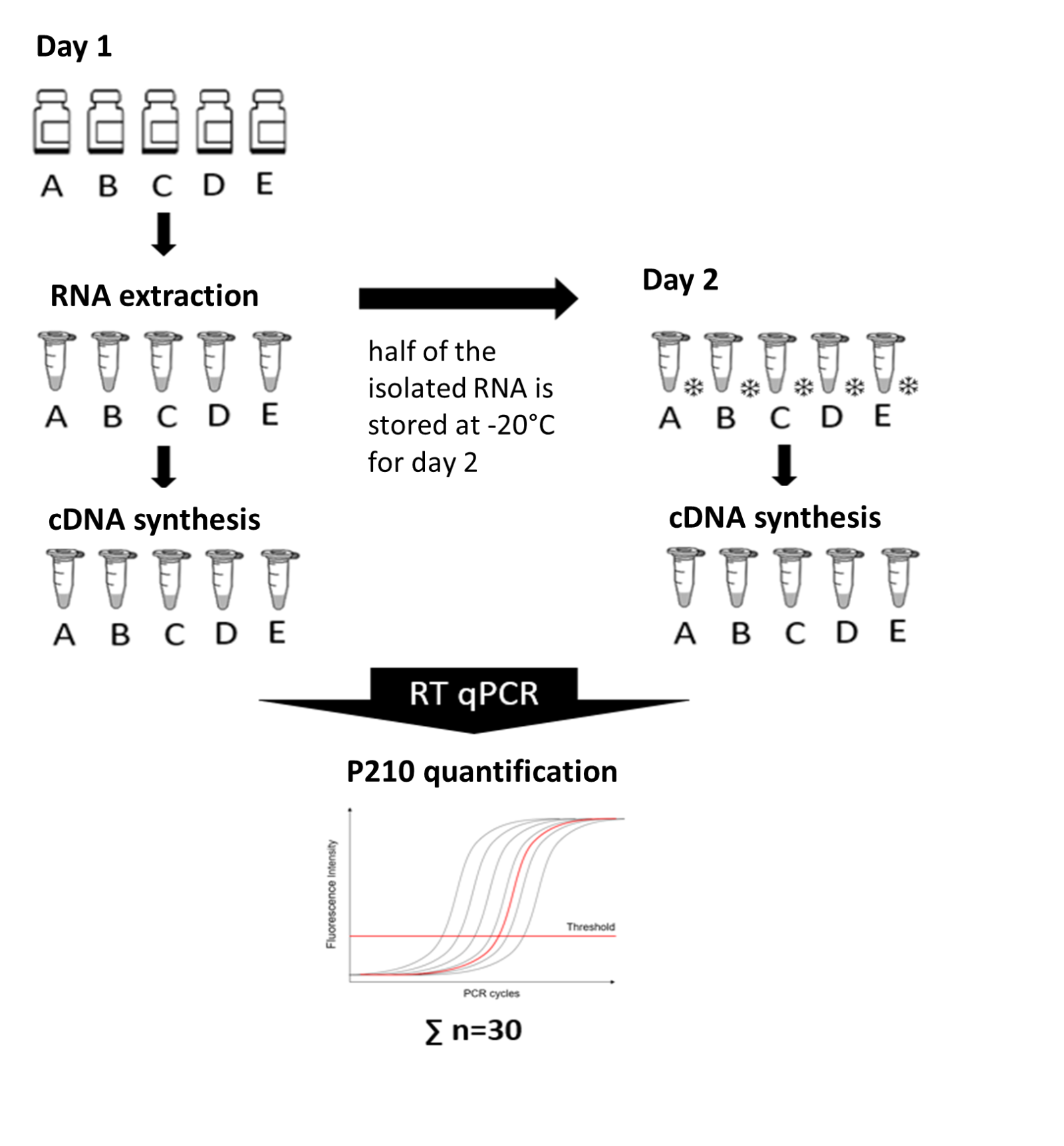
The EUTOS consortium provides a calculation template for determining the conversion factor according to the criteria on its website (https://www.uniklinikum-jena.de/eutos/Downloads.html). The individual conversion factor is determined by entering the measured values. In the future, this calculation will replace the certificate issued annually by EUTOS.
The newly validated conversion factor will be published on the MLL homepage, after which it can be used to calculate the BCR::ABL1IS for the CML progressions for one year.
Die Autorin

»Do you have questions regarding this article or do you need further information? Please send me an e-mail.«
Dr. rer. nat. Sandra Weißmann
Biologist, Dipl.
Head of PCR/Fragment Analysis
Deputy Head of Diagnostic reporting
sandra.weissmann@mll.com