MDS-Associated Aberrant Phenotypes in Multiple Myeloma
It has been known for about a decade that in some patients with multiple myeloma (MM), MDS-associated changes in the bone marrow can be detected at the time of diagnosis or later during the course of disease. These include genetic alterations, such as evidence of clonal hematopoiesis and/or MDS-associated cytogenetic anomalies, as well as aberrant immunophenotypes typical of MDS1. A recent study underlines the clinical relevance of such MDS-associated (immuno)phenotypic anomalies (MDS-PAs) and indicates
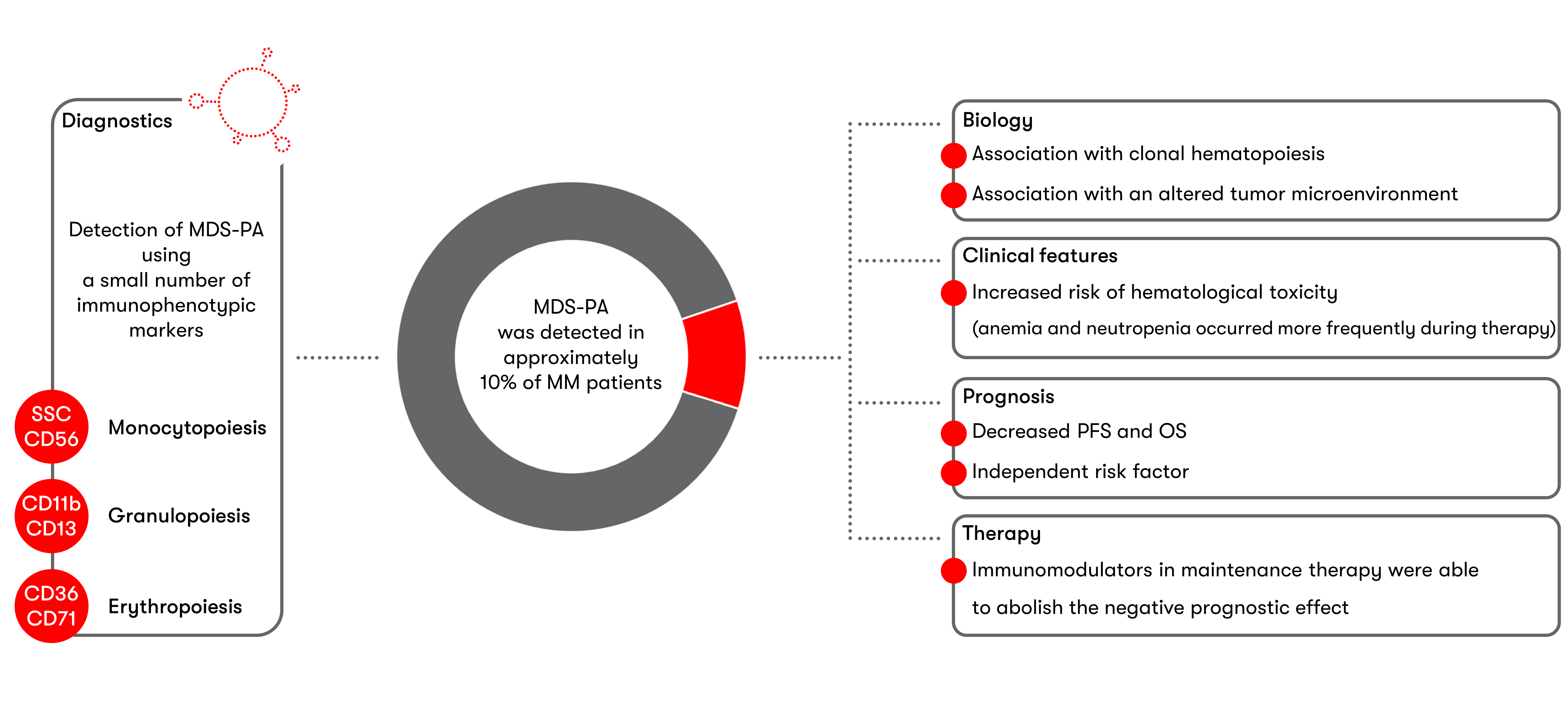
Following the initial literature reports2 on the use of flow cytometry to detect MDS-associated Phenotype Alterations (MDS-PAs) in multiple myeloma, a current study (Maia et al. Blood 2020) now deals with the biological, clinical, prognostic and therapy implications of MDS-PAs. Data were collected at the time of diagnosis and after high-dose therapy and autologous stem cell transplantation (HDT/ASCT).
Immunophenotypic characterization at the time of diagnosis was carried out in a group of 285 patients who participated in a therapeutic study. Characteristic MDS alterations were detected in bone marrow aspirates of 33 cases (11.6%), which most frequently included granulocytic dysplasia (22 patients), followed by erythroid dysplasia (10 patients) and monocytic dysplasia (7 patients). More than one lineage was affected in only 5 cases. However, even in cases with MDS-PAs, bone marrow smears displayed unremarkable morphology. In terms of biology, there was an association with an altered tumor microenvironment as well as clonal hematopoiesis. Molecular genetic analyses of 67 patients detected clonal hematopoiesis in 50% of MDS-PAs cases, but only in 22% of cases without MDS-PAs. Phenotype alterations and/or clonal hematopoiesis detected at diagnosis persisted in the majority of patients during the course of the disease; de novo MDS-PAs or somatic mutations after HDT/ASCT appeared in only a small percent of cases.
To evaluate the clinical consequences of MDS-PAs, a larger group of 1,252 patients selected from a total of four studies was examined. Since only data on CD56 expression were collected across all study cohorts, MDS-PA detection was restricted to the monocyte lineage. MDS-PAs could be detected at the time of diagnosis in 70 patients (5.6%). Patients with MDS-PAs had an increased risk of treatment-related hematologic toxicity and displayed anemia and neutropenia significantly more frequently than patients without MDS-PAs. MDS-PAs were also associated with decreased progression-free survival and overall survival. MDS-PAs turned out to be a risk factor that was independent of established risk parameters such as ISS stage III, high LDH and high-risk chromosome anomalies (cytogenetics). The negative prognostic effect on survival could, however, be counteracted by post-ASCT maintenance therapy with immunomodulatory drugs.
In summary, screening by flow cytometry proposed by Maia et al. is a cost-effective and rapid method for identifying patients with dysplastic hematopoiesis as early as the time of diagnosis. According to the authors, this may be particularly useful for MM patients with cytopenia of unknown etiology. The increased risk of therapy-related hematologic toxicity and the negative prognostic effect, which can be counteracted therapeutically by administering immunomodulators, make MDS-PA diagnostics clinically relevant.
References
1Barlogie et al. Blood 2008, Usmani et al. Blood 2013, Chitre et al. Leukemia 2018
2Matarraz et al. Haematologica 2012, Matarraz et al. Leukemia 2014