BCR::ABL1-negative myeloproliferative neoplasms (MPN) - overview
- Method:
- Anticoagulant:
- Recommendation:
- Method:Cytomorphology
- Anticoagulant:EDTA
- Recommendation:obligatory
- Method:Immunophenotyping
- Anticoagulant:EDTA or Heparin
- Recommendation:facultative
- Method:Chromosome analysis
- Anticoagulant:Heparin
- Recommendation:obligatory
- Method:FISH
- Anticoagulant:EDTA or Heparin
- Recommendation:facultative
- Method:Molecular genetics
- Anticoagulant:EDTA or Heparin
- Recommendation:obligatory
Myeloproliferative neoplasms (MPN) are rare, clonal diseases of the hematopoietic stem cell, which have many similarities. Especially in the early stages, they are often difficult to distinguish from each other and can also merge in individual cases. According to the WHO classification 2022, polycythemia vera (PV) shows an overall incidence of 1.57/100,000 person-years, essential thrombocythemia (ET) an overall incidence of 1.55/100,000 person-years, and primary myelofibrosis (PMF) in the fibrotic stage an annual incidence of 0.44-1.5 cases per 100,000 person-years (WHO 2022). MPNs usually affect people of older age with a median of 60-65 years (Barbui 2012). Characteristic of MPN is hypercellularity of the bone marrow, specifically the myeloid cell series, and increased numbers of erythrocytes, granulocytes, and/or platelets in the peripheral blood, depending on the entity (Haferlach et al. 2008, WHO 2022).
Myeloproliferative neoplasms: Classification
Classification into BCR::ABL1-positive CML and BCR::ABL1-negative myeloproliferative neoplasms is based on the WHO classification (Table 1):
Table 1: MPN WHO classification 2022 (WHO 2022)
|
Myeloproliferative neoplasms (MPN) |
|
|
Chronic myeloid leukemia (CML) |
BCR::ABL1 positive |
|
Polycythemia vera (PV) |
BCR::ABL1 negative |
|
Essential thrombocythemia (ET) |
|
|
Primary myelofibrosis (PMF) |
|
|
Chronic neutrophilic leukemia (CNL) |
|
|
Chronic eosinophilic leukemia (CEL) |
|
|
Juvenile myelomonocytic leukemia (JMML) |
|
|
Myeloproliferative neoplasms, not otherwise specified (NOS) |
|
Myeloproliferative neoplasms: Diagnostic methods and their relevance
Myeloproliferative neoplasms: Prognosis
In addition to increased age, leukocytosis, and thrombosis (Gangat et al. 2011, Barbui et al. 2018), in general, the detection of chromosomal alterations at diagnosis of an MPN seems to be associated with a less favorable prognosis. The presence of complex karyotypes during the course of an MPN increases the likelihood of transition to blast phase (Haferlach et al. 2008). SNP array analyses showed clustered deletions of the genes ETV6 (Chr. 12), TP53 (Chr. 17), or RUNX1 (Chr. 21) in blast phase (Thoennissen et al. 2010).
Gene mutations influence the risk profile of "classic" BCR::ABL1-negative MPNs
The JAK2 V617F mutation is the most common mutation in MPN. PV is almost always associated with this mutation (98%). In addition to the JAK2 V617F mutation, mutations in the CALR (20-30%) and MPL (5-10%) genes often occur in ET and PMF (Luque Paz et al. 2023). These mutations are among the driver mutations in MPN, but do not allow specific diagnosis due to their cross-entity occurrence.
81% of PMF patients, 53% of PV patients, and 53% of ET patients show mutations in addition to driver mutations (non-driver mutations, Fig. 1). IDH2 mutations are classified as a risk factor in all three classic BCR::ABL1-negative MPN (Tefferi et al. 2016, Tefferi & Vannucchi 2017). Furthermore, each of the three entities has an entity-specific risk profile (Fig. 1).
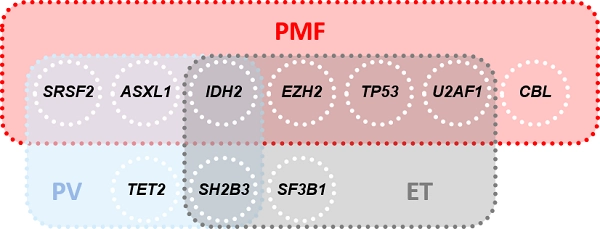
For a detailed insight into the individual MPNs and their genetic risk profiles, please refer to the infotexts of the respective entity:
Status: Dezember 2023