Diffuse large B-cell lymphoma (DLBCL)
- Method:
- Anticoagulant:
- Recommendation:
- Method:Cytomorphology
- Anticoagulant:EDTA
- Recommendation:obligatory
- Method:Immunophenotyping
- Anticoagulant:EDTA or Heparin
- Recommendation:obligatory
- Method:Chromosome analysis
- Anticoagulant:Heparin
- Recommendation:facultative
- Method:FISH
- Anticoagulant:EDTA or Heparin
- Recommendation:obligatory
- Method:Molecular genetics
- Anticoagulant:EDTA or Heparin
- Recommendation:facultative
Diffuse large B-cell lymphoma is the most common lymphoma in adults. We have summarized the most important information on (sub-) classification, diagnosis and prognosis. In addition, we provide further links on prognosis and therapy in DLBCL, so that you can inform yourself in more detail.
DLBCL: Classification
According to WHO classification 2022, diffuse large B-cell lymphoma, not elsewhere classified (DLBCL, NOS) is assigned to the mature B-cell neoplasms and here to the subgroup of large B-cell lymphomas. In addition to DLBCL, NOS, the WHO classification includes other distinct DLBCL entities. Defining criteria for these are localization as well as association to chronic inflammation or to specific viruses (EBV, KSHV/HHV8). ). In DLBCL, NOS, MYC, BCL2 or BCL6 translocations can occur which play an important role in the differential diagnosis. If a MYC and a BCL2 rearrangement are detectable simultaneously, a diffuse large B-cell lymphoma/highly malignant B-cell lymphoma with MYC and BCL2 rearrangements (DLBCL/HGBL-MYC/BCL2) should be diagnosed. In the presence of both MYC and BCL6 rearrangements, cytomorphology is critical for classification as DLBCL, NOS or HGBL, NOS (WHO 2022).
DLBCL: Subclassification
Morphological:
Based on morphology, DLBCL, NOS can be divided into: centroblastic, immunoblastic, anaplastic and rare variants. With around 80% of cases, the centroblastic subtype is the most common (WHO 2022).
Using gene expression profiles:
COO subtype: Subtyping according to cell of origin (COO) is important for the exact classification according to WHO and due to its prognostic relevance. The GCB subtype (germinal center B cells, GCB) and the ABC subtype (activated B-cell, ABC) are distinguished (Alizadeh et al. 2000). Approximately 10-15% of cases cannot be assigned to a COO subtype (Onkopedia-Leitlinie 2024.
Double-expressor phenotype: The so-called double-expressor phenotype is defined by the simultaneous expression of the MYC and BCL2 genes and is considered an established prognostic risk factor (Pasqualucci & Dalla-Favera 2018, WHO 2022). Approximately 2/3 of patients with double-expressing phenotype have ABC DLBCL (Hu et al. 2013).
Double-hit gene signature: Associated with the GCB subtype is a gene expression profile similar to that of double-hit lymphoma (simultaneous presence of a MYC and a BCL2 rearrangement) and therefore referred to as "double-hit signature" (DHITsig). DHITsig DLBCL can be distinguished from double-hit lymphomas by the absence of co-existing MYC and BCL2 rearrangements. DHITsig-positive GCB-DLBCL shows a worse prognosis compared with GCB cases without this gene expression signature (Ennishi et al. 2019).
Molecular high-grade: Another gene expression profile associated with GCB subtype has features of DLBCL and Burkitt lymphoma and is classified as "molecular high-grade" (Sha et al. 2019).
Molecular subclassification
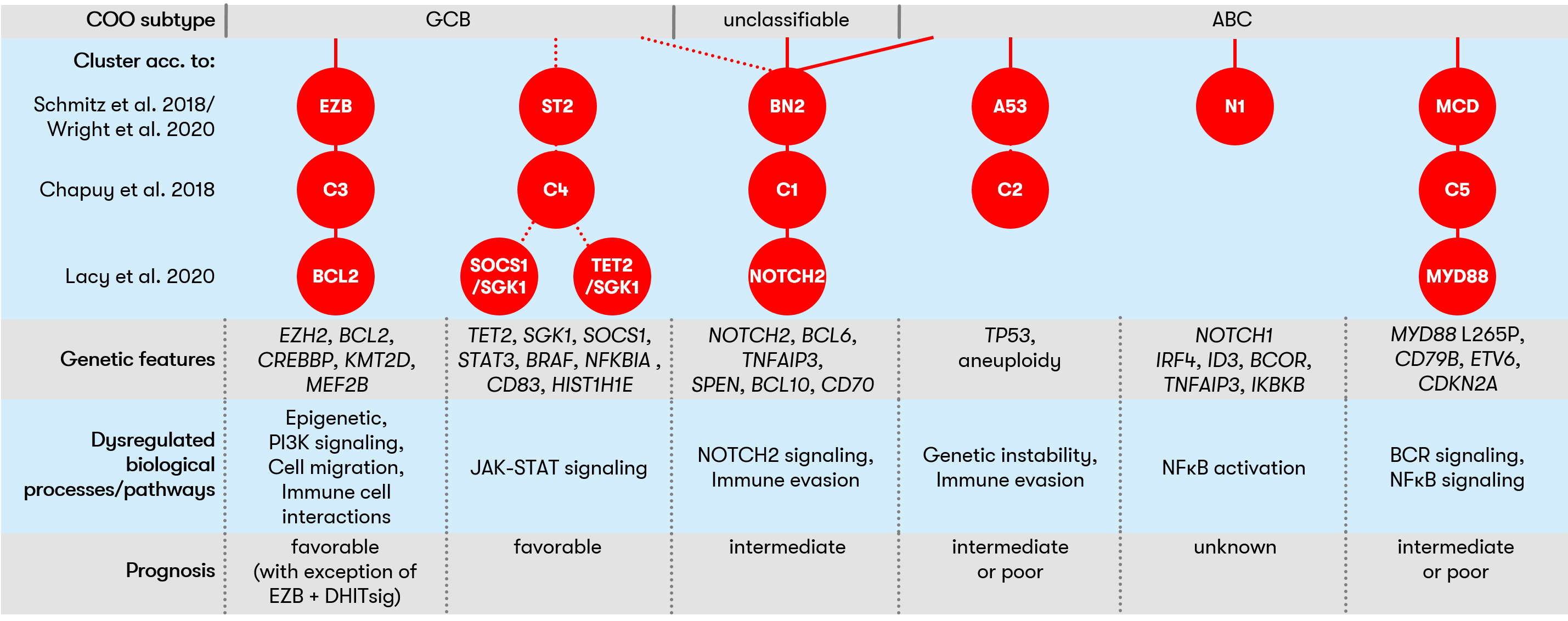
DLBCL is a genetically heterogeneous disease. Nevertheless, several independent studies showed that DLBCL can be classified into different clusters based on molecular signatures (Chapuy et al. 2018, Schmitz et al. 2018/Wright et al. 2020, Lacy et al. 2020). This classification allowed fine-grained risk stratification in each of the different studies and could also be of interest for the development of personalized therapies in the future.
Figure 1 provides an overview
of the clusters described and shows the molecular signatures associated with
each.
DLBCL: Diagnostic methods and their relevance
DLBCL: Prognosis
Gene expression profile influences prognosis
The classification of DLBCL according to COO subtypes is of prognostic and predictive relevance, as the two subtypes differ significantly in their response to the R-CHOP regimen. In this regard, the prognosis in GCB-DLBCL (with a 5-year survival of 80%) is better than in ABC-DLBCL (5-year survival of 50%) (Pon & Marra 2016). Simultaneous expression of MYC and BCL2 (so-called double-expressor phenotype) is associated with an unfavorable prognosis (Pasqualucci & Dalla-Favera 2018, WHO 2022). In addition, according to recent findings, a "double-hit" gene signature (Ennishi et al. 2019) or a "molecular high-grade" gene expression profile (Sha et al. 2019) also occur.
Clinical risk scores in DLBCL
The "International Prognostic Index (IPI)" takes into account age, lactate dehydrogenase level, Ann Arbor stage III or IV, and number of extranodal cases. Each of the above parameters can be assigned a risk point. The IPI was introduced before the rituximab era (Shipp et al. 1993). Further developments of the IPI are the "revised IPI" (R-IPI), and the NCCN-IPI. The R-IPI also takes into account performance status (ECOG 0-1 and 3-5) (Sehn et al. 2007). The NCCN-IPI, on the other hand, is more fine-grained for the factors age and LDH level, and the weighting also differs compared to the IPI or R-IPI. In addition, for extranodal involvement, localization rather than number is considered (Zhou et al. 2014). In a comparison of the three clinical scores, the NCCN-IPI possessed the greatest discriminatory power (Ruppert et al. 2020).
DLBCL: Prognosis calculation
DLBCL: Therapy
In first-line therapy, the R-CHOP regimen or R-CHOP-like protocols are used. For patients at increased risk (IPI 2-5), the guideline recommends 6 doses of R-CHP in combination with polatuzumab vedotin followed by two applications of rituximab. Innovative treatment concepts are becoming increasingly important in second-line therapy (Onkopedia Leitlinie 2024). The therapy algorithm according to the German guideline can be found on the Onkopedia page.
Status: April 2024