Myelodysplastic neoplasms (MDS)
- Method:
- Anticoagulant:
- Recommendation:
- Method:Cytomorphology
- Anticoagulant:EDTA
- Recommendation:obligatory
- Method:Immunophenotyping
- Anticoagulant:EDTA or Heparin
- Recommendation:facultative
- Method:Chromosome analysis
- Anticoagulant:Heparin
- Recommendation:obligatory
- Method:FISH
- Anticoagulant:EDTA or Heparin
- Recommendation:facultative
- Method:Molecular genetics
- Anticoagulant:EDTA or Heparin
- Recommendation:obligatory
Based on the current guidelines and the current state of research, there are different diagnostic recommendations for patients with myelodysplastic neoplasm. We have summarized the most important information on classification and diagnostic methods at MLL. In addition, we provide further links on prognosis and therapy in myelodysplastic neoplasm, so that you can inform yourself in more detail.
MDS: Classification
Myelodysplastic neoplasms (MDS, formerly: myelodysplastic syndromes) are acquired clonal bone marrow diseases that occur preferentially in older age. MDS is characterized by a proliferative and apoptotic pathology of hematopoietic progenitor cells. At the same time, an inflammatory microenvironment of the hematopoietic stem cell niche is often present, associated with increasing genome instability (Stubbins et al. 2022). Consequence is often anemia and also neutropenia and/or thrombocytopenia. Signs of dysplasia are seen in at least one of the three hematopoietic cell lineages, and leukemic transformation to AML is commonly found. Genetic characterization is playing an increasingly important role in diagnosis, which is reflected in the new WHO classification published in 2022. In this classification, "MDS with defining genetic abnormalities" and "MDS, morphologically defined" are now distinguished (WHO 2022):
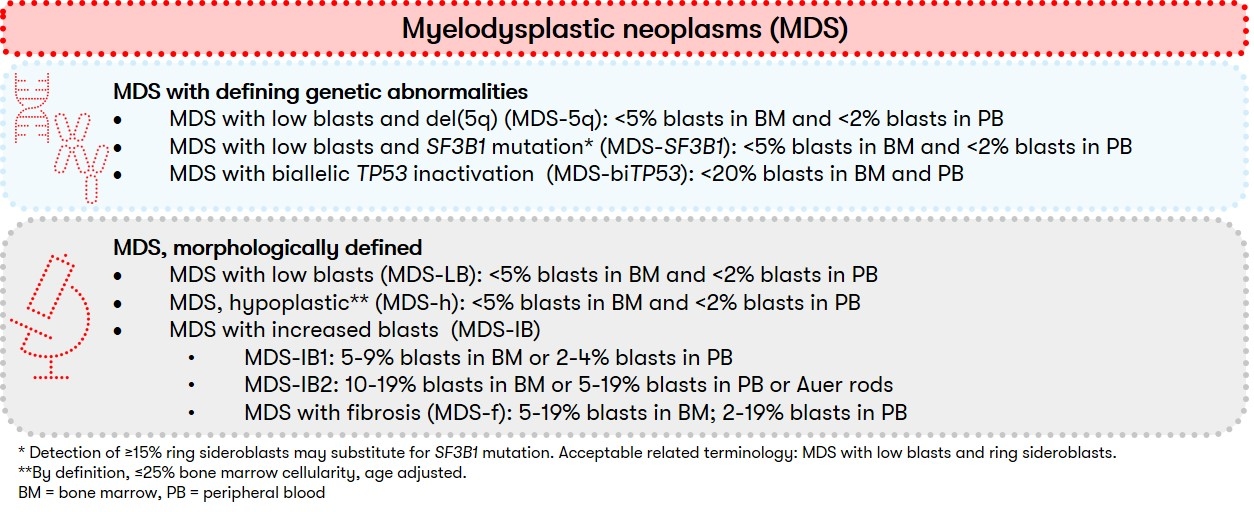
The new subgrouping is intended to provide a more accurate classification and thus the basis for targeted therapies. Since the number of dysplastic cell lines is usually dynamic and does not necessarily define a specific MDS type, the distinction between single and multilineage dysplasia is now optional according to WHO. In addition, within "MDS, morphologically defined," "MDS, hypoplastic (MDS-h)" represents a newly defined subgroup with cytopenia, dysplasia, and strongly reduced bone marrow cellularity. The subgroup of "MDS, unclassifiable" is no longer used in the new WHO classification.
MDS: Diagnostic methods and their relevance
MDS: Prognosis
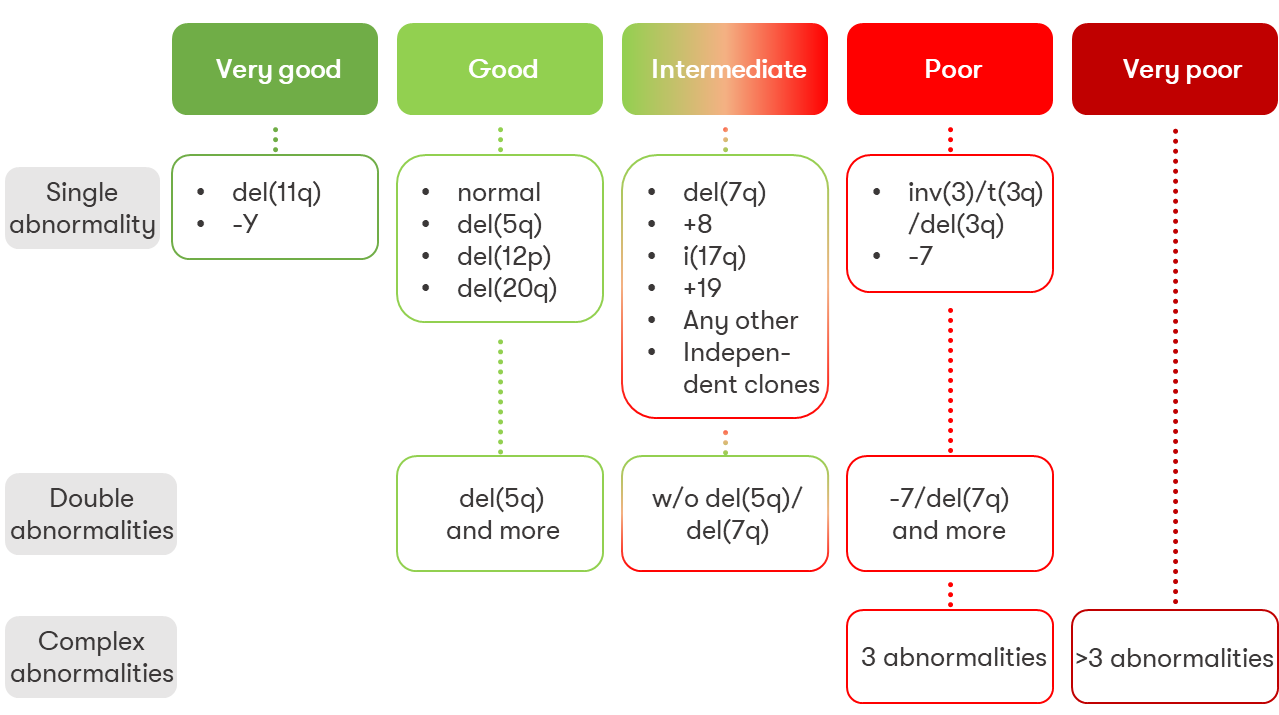
For many years, the International Prognostic Scoring System (IPSS) (Greenberg et al. 1997) was the mainstay of prognostic classification for patients with MDS. For improved or more detailed risk stratification of patients with MDS, the IPSS was revised in 2012 (Revised-IPSS, IPSS-R) (Greenberg et al. 2012). The now strong emphasis on molecular genetics is reflected in a new prognostic score (IPSS-M), which takes into account primarily molecular genetic findings in addition to clinical and cytogenetic ones. In this new system, prognosis assessment is based on hemoglobin level, platelet count, bone marrow blast count, cytogenetic prognostic category (as in IPSS-R), and molecular genetic information on 31 genes. A summary of the information can be found in a review article on our website (Bernard et al. 2022). Click here for the prognosis calculation of the IPSS score, the IPSS-R score, WPSS score and the new IPSS-M taking into account the molecular genetic results.
MDS: Recommendation & Therapy
Cytomorphological bone marrow diagnostics in combination with cytogenetics and molecular genetics represent the current gold standard in MDS diagnostics (Bernard et al. 2022). Current studies can be accessed via the MDS Patient Portal and the German MDS Study Group.
Since MDS are considered dynamic diseases, the Onkopedia guideline advocates annual screening in the areas of cytomorphology, cytogenetics, FISH, and molecular genetics - especially if risk stratification can be expected to change in an individual case (Onkopedia Guideline MDS 2023).
Therapy of myelodysplastic neoplasm according to the German guideline depends, among other things, on the risk group as well as the age and clinical condition of the patient (Onkopedia Guideline MDS).
Status: September 2023