Waldenström Macroglobulinemia / Lymphoplasmacytic lymphoma (LPL)
- Method:
- Anticoagulant:
- Recommendation:
- Method:Cytomorphology
- Anticoagulant:EDTA
- Recommendation:obligatory
- Method:Immunophenotyping
- Anticoagulant:EDTA or Heparin
- Recommendation:obligatory
- Method:Chromosome analysis
- Anticoagulant:Heparin
- Recommendation:facultative
- Method:FISH
- Anticoagulant:EDTA or Heparin
- Recommendation:facultative
- Method:Molecular genetics
- Anticoagulant:EDTA or Heparin
- Recommendation:obligatory
Waldenström macroglobulinemia is a rare lymphoproliferative disorder characterized by the excessive production of IgM monoclonal antibodies. Based on the current guidelines and the current state of research, there are different diagnostic recommendations for patients with Waldenström macroglobulinemia. We have summarized the most important information on classification and diagnostic methods at the MLL. In addition, we provide further links on prognosis and therapy in Waldenström macroglobulinemia.
Waldenström macroglobulinemia: Classification
Waldenström macroglobulinemia is a rare chronic lymphoproliferative disorder that is usually indolent. Only 10-15% of patients show more rapid progression of the disease. According to the WHO classification of 2022, Waldenström macroglobulinemia is classified as mature B-cell neoplasms and here assigned to lymphoplasmacytic lymphomas (LPL). WHO distinguishes 2 subtypes of LPL, of which Waldenström macroglobulinemia / IgM-LPL is the far more common. Non- Waldenström macroglobulinemia LPL account for only about 5% of LPL. They include cases with IgG or IgA monoclonal proteins, non-secretory LPL, and IgM-LPL without bone marrow involvement (WHO 2022).
Characteristic and diagnosis-defining features are a lymphoplasmacytic infiltration of the bone marrow and a monoclonal immunoglobulin M (IgM). The origin of the pathological cell population are probably B cells that have not yet undergone an isotype change but have already undergone the germinal center reaction.
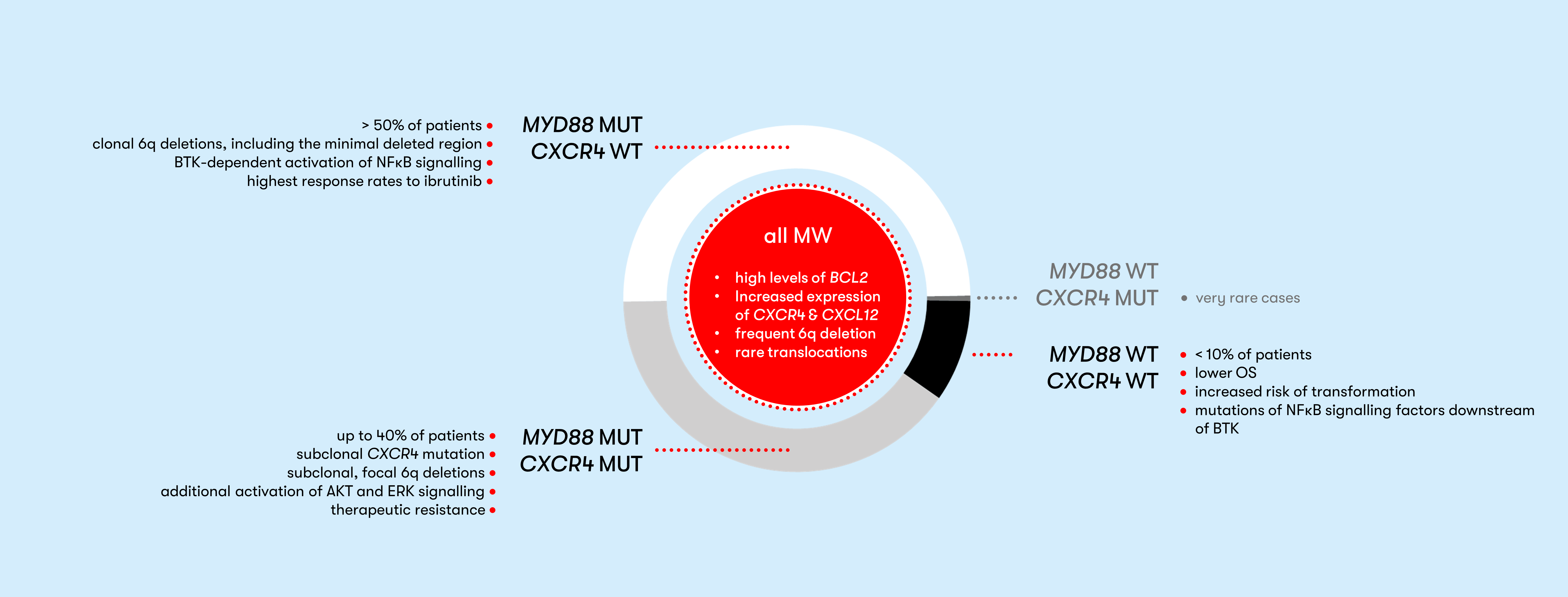
The laboratory diagnosis of IgM-MGUS (monoclonal gammopathy of undetermined significance) develops into Waldenström macroglobulinemia at a progression rate of 1.5-2% per year (Kyle et al. 2003, Bustoros et al. 2019). IgM-MGUS is defined by a serum monoclonal IgM concentration below 30 g/l and less than 10% plasma cells in the bone marrow (WHO 2022). 6q deletions that are characteristic of Waldenström macroglobulinemia (see also chromosomal analysis) represent a risk factor for transformation of IgM-MGUS into Waldenström macroglobulinemia (Paiva et al. 2015, Guerrera et al. 2018).
Table 1: Classification of Waldenström´s macroglobulinemia and related disorders according to the European Consortium for Waldenström´s Macroglobulinemia (Dogliotti et al. 2023)
|
|
IgM monoclonal protein |
Bone marrow infiltration |
Symptoms attributable to IgM |
Symptoms due to tumor infiltration |
|
Symptomatic WM |
+ |
+ |
+ |
+ |
|
Asymptomatic WM |
+ |
+ |
- |
- |
|
IgM-related disorders |
+ |
- |
+ |
- |
|
IgM-MGUS |
+ |
- |
- |
- |
Waldenström macroglobulinemia: Diagnostic methods
Waldenström macroglobulinemia: Prognosis
A risk assessment for prognosis can be made with the two scores „International scoring system for Waldenström’s macroglobulinemia“ (ISSWM) (Morel et al. 2009) and „revised international prognostic score system for Waldenström’s macroglobulinemia“ (rIPSSWM) (Kastritis et al. 2019).
Prognostically relevant gene mutations or chromosomal aberrations are not considered in either score.
Waldenström macroglobulinemia: Therapy
Up to 30% of patients have no symptoms at diagnosis. This group is classified as asymptomatic or smoldering Waldenström macroglobulinemia (SMW) (Pophali et al. 2019). The rate of progression to symptomatic Waldenström macroglobulinemia is 12% per year (Bustoros et al. 2019). For these patients, there is a need for close monitoring. The risk for progression to symptomatic Waldenström macroglobulinemia can be determined using an online tool. Relevant to treatment response are CXCR4 mutations, which can negatively affect response to BTK inhibitors in MYD88 L265P mutated patients (Treon et al. 2015, Treon et al. 2018, Tam et al. 2020). In symptomatic patients, genetic characterization plays an increasingly important role in therapy selection. An overview of therapy algorithms can be found in the Onkopedia guideline Waldenström macroglobulinemia (lymphoplasmacytic lymphoma) and in a clinical update by M. Gertz (Gertz 2023). In addition, an overview of therapy management with BTK inhibitors is provided by Buske et al. (Buske et al. 2023).
Status: August 2023