Mastocytosis
- Method:
- Anticoagulant:
- Recommendation:
- Method:Cytomorphology
- Anticoagulant:EDTA
- Recommendation:obligatory
- Method:Immunophenotyping
- Anticoagulant:EDTA or Heparin
- Recommendation:obligatory
- Method:Chromosome analysis
- Anticoagulant:Heparin
- Recommendation:obligatory
- Method:FISH
- Anticoagulant:EDTA or Heparin
- Recommendation:facultative
- Method:Molecular genetics
- Anticoagulant:EDTA or Heparin
- Recommendation:obligatory
Mastocytosis is characterized by an accumulation of neoplastic mast cells in one or more organs. It is a heterogeneous disease ranging from skin lesions to aggressive haematologic neoplasms. Based on the current guidelines and the current state of research, different diagnostic recommendations emerge. We have summarized the most important information about classification and diagnostic methods at the MLL. In addition, we have compiled further literature and links on prognosis and therapy in mastocytosis, so that you can inform yourself in more detail.
Mastocytosis: Classification and staging
According to WHO, mastocytosis can be classified into three major categories (Tab. 1). Cutaneous and systemic mastocytosis can each be further divided into subgroups. While in cutaneous mastocytosis the mast cells accumulate in the skin, the systemic variant involves at least one extracutaneous organ, almost always infiltrating the bone marrow. The very rare mast cell sarcoma is a solid tumor consisting of atypical malignant mast cells.
Tab. 1: WHO classification of mastocytosis (WHO 2022)
|
Cutaneous mastocytos (CM) |
|
|
Systemic mastocytosis (SM)* |
* Well-differentiated systemic mastocytosis (WDSM) represents a morphologic variant that can occur in any SM type/subtype, including mast cell leukemia. |
|
Mast cell sarkoma |
|
Tab. 2: Diagnostic criteria according to WHO 2022 (WHO 2022)
|
Cutaneous mastocytosis |
Essential:
Desirable:
|
|
Systemic mastocytosis |
The diagnosis of systemic mastocytosis can be made when the major criterion and at least 1 minor criterion are present, or when >3 minor criteria are present. Major criterion: Multifocal dense infiltrates of mast cells (> 15 mast cells in aggregates) detected in sections of bone marrow and/or other extracutaneous organ(s) Minor criteria: 1. >25% of all mast cells are atypical cells (type I or type II) on bone marrow smears or are spindle-shaped in dense and diffuse mast cell infiltrates in sections of BM or other extracutanous organ(s) (in the KM smear, atypical mast cell morphology does not count as a SM criterion if the mast cells are located in or adjacent to bone marrow particles). 2. Evidence of activating KIT D816 point mutation(s) or in other critical regions of KIT in bone marrow or other extracutaneous organs (any KIT mutation is considered a secondary criterion if there is published evidence of its transforming behavior). 3. Mast cells in bone marrow, blood, or another extracutaneous organ(s) aberrantly express one or more of the following antigens: CD2, CD25, CD30 (confirmed by flow cytometry or immunohistochemistry). 4. Baseline serum tryptase concentration >20 ng/mL in the absence of a myeloid AHN (in the presence of associated myeloid neoplasia, this criterion is not suitable for the diagnosis of systemic mastocytosis; in the presence of hereditary α-tryptasemia, correction based on the number of additional α-tryptase gene copies is recommended). |
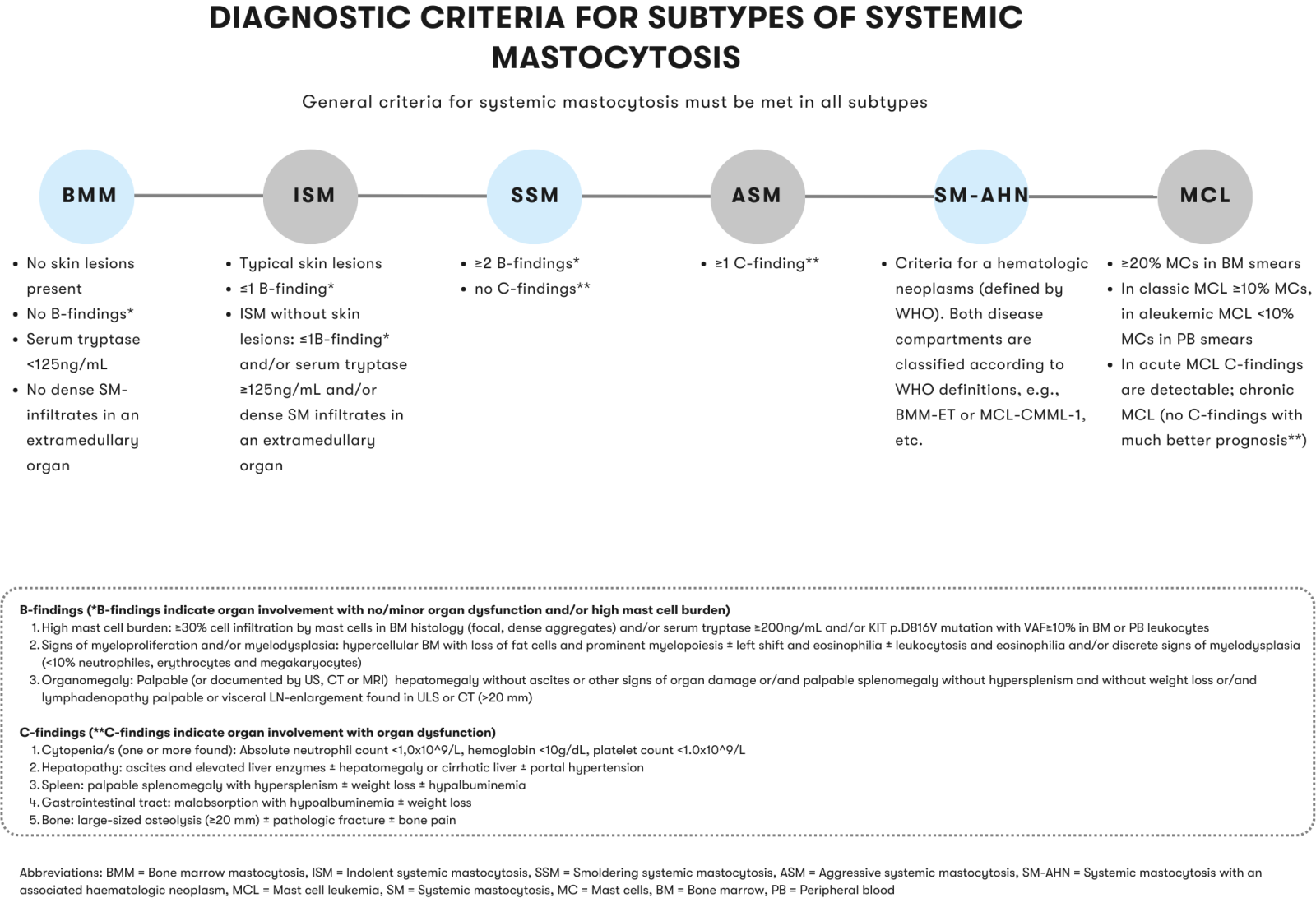
A practical algorithm for the diagnosis and classification of systemic mastocytosis is provided by the International Consensus Classification of Eosinophilic Diseases and Systemic Mastocytosis (Wang et al. 2023) and by H. J. Lee (Lee et al. 2023).
Mastocytosis: Diagnostic methods and their relevance
Mastocytosis: Prognosis and Therapy
Cutaneous mastocytosis usually has a favorable course. It occurs more frequently in childhood. In most cases, the skin lesions regress on their own by adulthood. If it breaks out in adulthood, it is closely associated with systemic involvement (Berezowska et al. 2014, Valent et al. 2017, WHO 2022, Valent et al. 2023).
Systemic mastocytosis develops almost exclusively in adulthood (Valent et al. 2023). The prognosis depends on the associated subgroup. ISM usually has a favorable course and patients have a normal life expectancy in most cases (WHO 2022). However, 5-10% of ISM show progression to advanced systemic mastocytosis and an associated less favorable prognosis. There are data suggesting that in ISM, in addition to the KIT mutation, additional mutations in the ASXL1, RUNX1, and/or DNMT3A genes (VAFs ≥30%) are associated with shorter progression-free and overall survival (Muñoz-González et al. 2019).
ASM, MZL, and mast cell sarcoma show an unfavorable prognosis (Lim et al. 2009, Monnier et al. 2016, WHO 2022). ASM, MZL, and SM-ANH are also grouped together as advanced systemic mastocytosis because of their generally unfavorable prognosis. For SM-AHN, the associated haematologic neoplasm and the associated cytogenetic and/or molecular genetic alterations should also be considered to assess prognosis (Wang et al. 2013, Naumann et al. 2018, WHO 2022). For cytogenetic alterations, classification into risk groups is possible depending on the associated haematologic neoplasm (Naumann et al. 2018).
Several prognostic scores have been published in recent years for both ISM and advanced SM individually and for SM as a whole: The International Prognostic Scoring System for Mastocytosis (IPSM) is based on simple clinical variables such as age, blood count changes, serum tryptase, and alkaline phosphatase (Sperr et al. 2019). The Mutation-Adjusted Risk Score for Advanced Systemic Mastocytosis (MARS) includes mutations in SRSF2, ASXL1, and RUNX1 in addition to age and blood count changes (Jawhar et al. 2019). The Global Prognostic Score (GPS) for SM similarly integrates additional mutations in the SRSF2, ASXL1, RUNX1, and DNMT3A genes with blood count alterations and serum biomarkers (Muñoz-González et al. 2021).
The therapeutic options strongly depend on the progress of the disease and comorbidities and should be considered individually. Studies by DeAngelo et al. and J. Gotlib et al. has led to approval of avapritinib in ASM, SM-AHN, and MCL (DeAngelo et al. 2021, Gotlib et al. 2021). An overview of therapy algorithms is among others given in A. Pardanani (Pardanani 2021), M. Sciumè et al. (Sciumè M et al.), the International Consensus Classification of Eosinophilic Diseases and Systemic Mastocytosis (Wang et al. 2023) and in the Onkopedia guideline systemic mastocytosis 2020.
Status: September 2023