BAX mutations mediate venetoclax resistance in AML
The development of targeted therapies for the treatment of hematological neoplasms has yielded a large number of promising drugs in recent years. Among these, substances that induce programmed cell death (apoptosis) by inhibiting anti-apoptotic proteins, e.g. BCL2, deserve special mention. Currently, the best-known representative of this substance class is venetoclax. However, as with many other targeted therapies, the problem of resistance formation during the course of treatment frequently arises.
Use of venetoclax in the treatment of AML
The use of venetoclax has particularly changed the therapy of AML patients who are not eligible for treatment with intensive high-dose chemotherapy. These patients are mostly elderly or unfit patients. The combination of venetoclax with hyomethylating agents or low-dose cytarabine has emerged as the standard treatment option for newly diagnosed AML.1,2. Since resistance and disease progression often occur during the course of treatment, the elucidation of the underlying mechanisms is the subject of intensive research.
Mechanisms of resistance formation
Already known mechanisms of resistance to venetoclax treatment include mutations in genes such as TP53 as well as K/NRAS. In addition, FLT3-ITD is also a corresponding risk factor.3,4. In contrast, mutations in BCL2, one of the "targets" of venetoclax, which are already known from studies on CLL, do not play a role.
A newly discovered candidate gene involved in AML is the proapoptotic BAX gene. This is reported in a recent publication in Blood by the group of Moujalled et al.5.
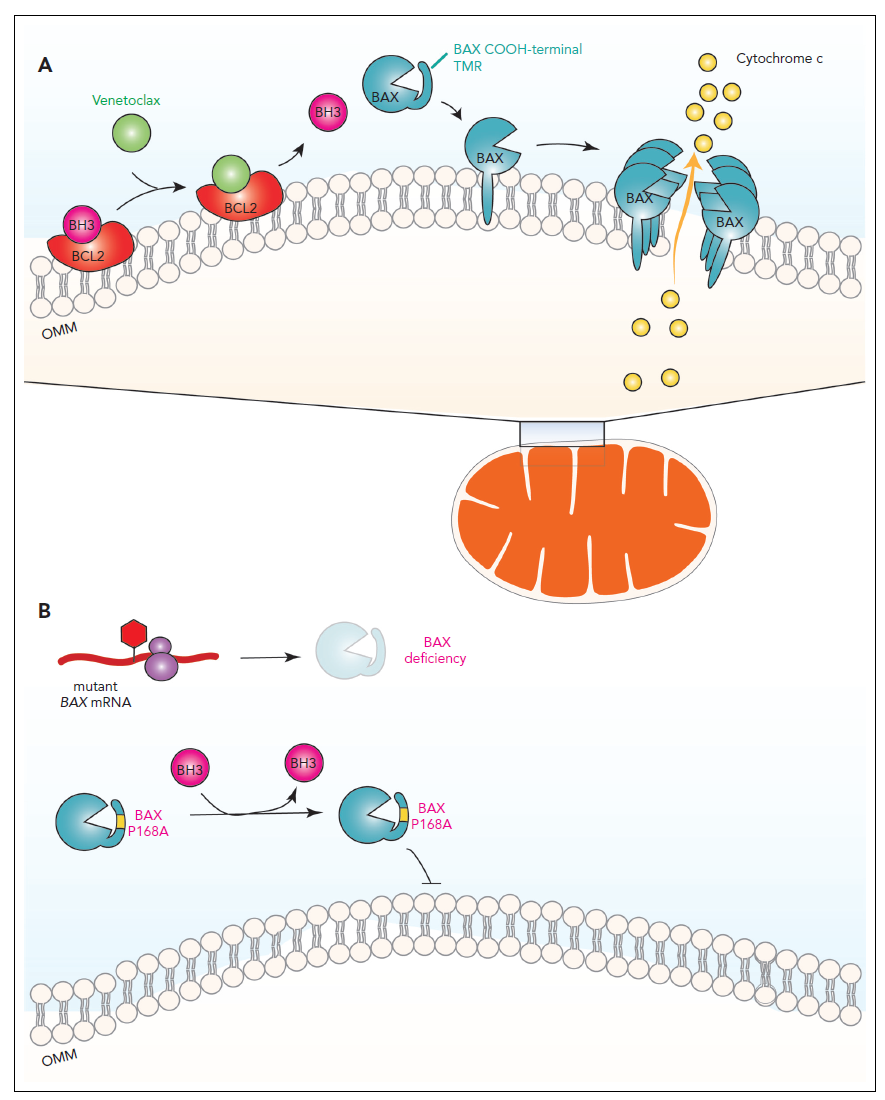
Apoptosis induction by venetoclax and inhibition of apoptosis by BAX mutations.
The mechanisms of apoptosis induction are shown in the figure (taken from Kim WJ et al, Blood 2023, Comment on Moujalled et al). Here, apoptosis is ultimately induced by the binding of BAX to the outer mitochondrial membrane. BAX-mediated permeabilization of the membrane occurs, which in turn leads to the release of cytochrome c from inside the mitochondria into the cytosol of the cell. Two steps are critical for the activation of BAX to bind to the outer mitochondrial membrane:
- BCL2 must be bound by a so-called proapoptotic "BH3 only" protein or by the BH3 mimetic venetoclax as an alternative ligand.
- Excess venetoclax, in addition to binding BCL2, simultaneously results in increased release of "BH3-only" proteins, which have as a second function the activation of BAX.
References
- DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N Engl J Med. 2020;383(7):617-629.
- Wei AH, Montesinos P, Ivanov V, et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood. 2020;135(24):2137-2145.
- DiNardo CD, Tiong IS, Quaglieri A, et al. Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood. 2020;135(11):791-803.
- Thijssen R, Diepstraten ST, Moujalled D, et al. Intact TP-53 function is essential for sustaining durable responses to BH3-mimetic drugs in leukemias. Blood. 2021;137(20):2721-2735.
- Moujalled DM, Brown FC, Chua CC, et al. Acquired mutations in BAX confer resistance to BH3-mimetic therapy in acute myeloid leukemia. Blood. 2023;141(6):634-644.
The author

»You have questions about the article or want more information? Please feel free to send me an e-mail.«
Dr. rer. nat. Frank Dicker